Abstract
Introduction Adult T-cell leukemia-lymphoma (ATL) is an aggressive hematological malignancy associated to poor outcomes. Most of these patients experience a rapid and aggressive disease course characterized by early progression and death. Previous studies have identified clinical and laboratory factors of prognostic value. However, their incorporation in the clinical setting has not been validated due to unstandardized management practices. Risk stratification based on time to progression has not been explored in patients with ATL. Therefore, we develop a clinical endpoint to evaluate whether patients that relapse early have a higher risk of death.
Methods We designed a multicenter retrospective cohort study among treated patients with a serological and pathology confirmation of ATL. Included patients with acute or lymphomatous ATL subtypes received systemic treatment (e.g., chemotherapy; zidovudine plus interferon). The training cohort consists of patients from endemic regions treated in tertiary care centers across Latin America and the United States. A validation set of 193 patients from Japan was used for reproducibility. Our exploratory analysis identified that at least 20% of relapsed patients experienced this event within 5 months after diagnosis in both training and validation cohorts. Therefore, we used a 5-month landmark for patients with ATL (ATL5) to define early progression. Median follow-up (reverse Kaplan-Meier) was 48 and 40 months in the training and validation cohorts, respectively. Overall survival was defined from date progression for early-ATL5, and from the 5-month landmark for non-early-ATL5 (reference group). Patients that died without relapse within 5 months (n=62 training, n=54 validation) or those alive with less than 5 months of follow-up time (n=13 training, n=3 validation) were excluded from the analysis. We plotted Kaplan-Meier curves and used the log-rank test to compare groups across the datasets. Univariate and multivariate Cox regression analyses were employed to model the effect of ATL5 on all-cause mortality. Missing values were added as a missing category in the adjusted models.
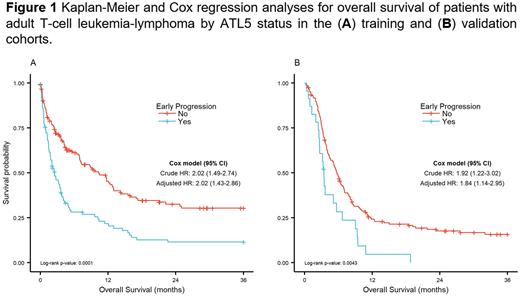
Results Of the 253 patients in the training cohort, 38% (n=96) relapsed within 5 months after diagnosis, and 62% (n=157) did not (reference group). There were 96 (38%) with acute (early-ATL5: n=55, reference: n=41) and 157 (62%) with lymphoma (early-ATL5: n=41, reference: n=116) ATL subtype. The median age at diagnosis was similar between both groups (early relapse: 55 years, reference: 54 years; p=0.831). Patients with early-ATL5 had poor performance status (ECOG 2-4 68% vs. 49%, p=0.009), higher frequency of acute ATL (57% vs. 26%, p<0.001), B symptoms (83% vs. 63%, p=0.034), advanced stage (III-IV 97% vs. 84%, p=0.002), hypercalcemia (51% vs. 35%, p=0.023), and higher values of lactate dehydrogenase (96% vs. 77%, p<0.001). Overall survival at 3 years was poorer among patients with early-ATL5 (12% vs. 30%; p=0.0001; Figure 1A). The univariate Cox analysis identified a hazard ratio (HR) of 2.02 (95% confidence interval [CI] 1.49-2.74) for early progression. After adjusting for ATL subtype, cancer stage, skin involvement, ECOG score, extranodal involvement, and hypercalcemia, patients with early-ATL5 remained with a higher risk of death (adjusted HR: 2.02, 95% CI 1.43-2.86). In the validation cohort, the 23 (12%) patients with early progression were deceased within 18 months after the landmark basal time (Figure 1B). The risk of death was higher among individuals with early-ATL5 in the validation set (adjusted HR: 1.84, 95% CI 1.14-2.95, Figure 1B).
Conclusions Patients with ATL who relapse within 5 months after diagnosis had a higher risk of death. Treatment of ATL varies between and within countries, limiting the comparison and validation of current prognostic factors. Therefore, we developed a time-based marker to evaluate the prognosis of these patients. Our findings remain robust in the validation set, suggesting that ATTL5 is a clinically relevant endpoint in the real-world setting. Future studies should validate its applicability in prospective studies and determine its relevance for risk-based treatment in clinical trials.
Disclosures
Utsunomiya:Bristol-Myers: Honoraria; Meiji Seika Pharma: Honoraria; JIMRO: Consultancy; Otsuka Medical Devices: Consultancy. Takamatsu:Janssen Pharmaceutical: Honoraria; AstraZeneca: Honoraria; Sanofi: Honoraria; Chugai Pharmaceutical: Research Funding. Ishitsuka:BMS: Honoraria; Chugai: Honoraria; Nippon-Shinyaku: Honoraria; Astellas: Honoraria; Ono: Honoraria, Research Funding; Celgene: Honoraria; Otsuka: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Yakult: Honoraria; AbbVie: Honoraria; CSL Behring: Honoraria; Sanofi: Honoraria; Otsuka: Honoraria; Pfizer: Honoraria; Meiji Seika: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; OQVIA: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Takeda: Honoraria. Suzumiya:Jansen: Honoraria; Eli Lill: Honoraria; Nihonshinyaku: Honoraria; SymBio: Honoraria; Otsuka: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria; Bristol Myers Suquibb: Honoraria; AstraZeneca: Honoraria; Eisai: Honoraria, Research Funding; Dainihon-Sumitomo: Honoraria; Chugai-Roche: Consultancy, Honoraria, Research Funding; Zenyaku: Consultancy; Novartis: Honoraria; Takeda: Honoraria. Tamura:AC Medical: Consultancy; Symbio Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Eisai Co., Ltd: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Ono Pharmaceutical Co., Ltd: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal